Trial specifications
Summary.
The TOGETHER Clinical Trial was initiated in June of 2020, as an international collaboration in response to the worsening pandemic of SARS-CoV-2 / COVID-19. Utilizing an adaptive platform trial design, the international researchers and teams in Brazil, Canada, Australia, and the United States sought to identify evidence-informed therapeutics, through the re-purposing of existing medications.
The randomized adaptive platform trial aims to investigate the efficacy of repurposed treatments for COVID-19 disease among high-risk adult outpatients. Currently, there are 22 identified research sites in Brazil, and over 6,000 research participants.
Table 1: Interventions investigated
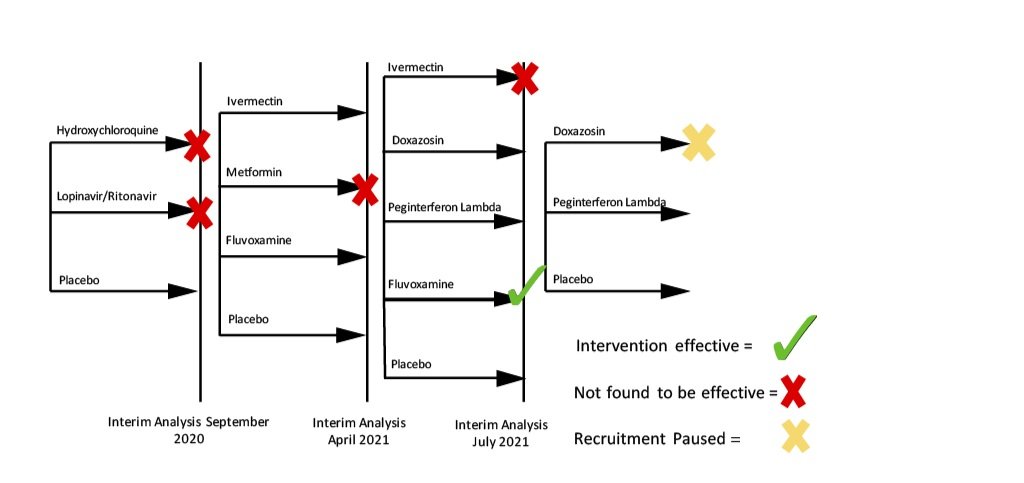
Trial Schema
Clinical trials
As of the most recent update, the TOGETHER Trial has concluded investigations on five medications (hydroxychloroquine, lopinavir / ritonavir, ivermectin, fluvoxamine maleate, and metformin), and two investigations (doxazosin and Peginterferon Lambda) are continuing (see Table 1). A summary of the progression of clinical trials is presented in Figure 1.
Recruitment for the Trial began in 2020 and has been ongoing to present day. Eligible patients are randomized with equal chance to an investigational product (IP) or to placebo. Patients are included if they are:
18 years of age
have a positive antigen test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
have an indication for high risk of disease severity, including co-morbidities, older age, or high body mass index.
Trial results
Click on the tabs below to read a summary of the following medication trials:
Publications
Click on the tab below for a list of relevant publications.
The TOGETHER Trials have received research ethics approval in both Brazil (CEP/CONEP#: 41174620.0.1001.5120) and in Canada (HiREB#: 13390). An independent data and safety monitoring committee is providing ongoing oversight of our work.